Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Maamoun, I.; Rushdi, M.*; Falyouna, O.*; Eljamal, R.*; Eljamal, O.*
Separation and Purification Technology, 308, p.122863_1 - 122863_16, 2023/03
Times Cited Count:3 Percentile:34.99(Engineering, Chemical) ]
] from hydrochloric acid using a simple urea extractant
from hydrochloric acid using a simple urea extractantUeda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Wenzel, M.*; Weigand, J. J.*; Oto, Keisuke*
Separation and Purification Technology, 277, p.119456_1 - 119456_8, 2021/12
Times Cited Count:5 Percentile:30.31(Engineering, Chemical)no abstracts in English
Hayashi, Natsuki*; Matsumura, Daiju; Hoshina, Hiroyuki*; Ueki, Yuji*; Tsuji, Takuya; Chen, J.*; Seko, Noriaki*
Separation and Purification Technology, 277, p.119536_1 - 119536_8, 2021/12
Times Cited Count:15 Percentile:62.75(Engineering, Chemical)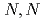 -dioctylacetamide and direct electrodeposition from loaded organic phase
-dioctylacetamide and direct electrodeposition from loaded organic phaseMatsumiya, Masahiko*; Song, Y.*; Tsuchida, Yusuke*; Sasaki, Yuji
Separation and Purification Technology, 234, p.115841_1 - 115841_8, 2020/03
Times Cited Count:17 Percentile:62.19(Engineering, Chemical)The development of solvent extraction and direct electrodeposition processes is an important task to reduce the volume of secondary wastes. In this study, the extraction of Pd(II) from hydrochloric/chloride media using methylimino-bis-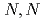 -dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
-dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
Maeda, Motoki*; Narita, Hirokazu*; Tokoro, Chiharu*; Tanaka, Mikiya*; Motokawa, Ryuhei; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation and Purification Technology, 177, p.176 - 181, 2017/04
Times Cited Count:22 Percentile:58.79(Engineering, Chemical)